Pharmaceutical Analytical Testing Market
Pharmaceutical Analytical Testing Market Share & Trends Analysis Report, By Service Type (Bioanalytical Testing, Method Development and Validation, Stability Testing, Drug Substances Testing, Microbial Testing, Physical Characterization, Others), By End User (Medical Device Companies, Pharmaceutical & Biopharmaceutical Companies, Others) – Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast, 2025–2033

Historical Period: 2019-2024
Forecast Period: 2025-2033
Report Code :
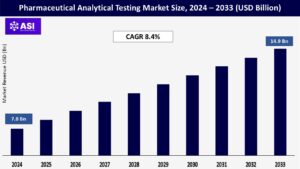
CAGR: 8.4%
Last Updated : August 22, 2025
The global Pharmaceutical Analytical Testing Market was valued at approximately USD 7.8 billion in 2024 and is projected to reach USD 14.9 billion by 2033, growing at a CAGR of 8.4% during the forecast period (2025–2033).
The Pharmaceutical Analytical Testing Market focuses on the systematic evaluation of pharmaceutical materials and products to confirm their quality, safety, effectiveness, and adherence to regulatory guidelines. This includes analyzing raw ingredients, active pharmaceutical compounds, final drug formulations, and their packaging. The market serves multiple purposes, such as quality assurance, stability assessments, validation of analytical methods, raw material verification, microbial testing, bioanalytical studies, and ensuring regulatory compliance and environmental hygiene in production areas. Known for its precision and reliability, this sector utilizes advanced technologies like HPLC, GC, MS, NMR, and UV spectroscopy. Operations are conducted under strict regulatory frameworks (e.g., USP, ICH) and increasingly involve outsourcing to Contract Research Organizations (CROs). A key emphasis is placed on data integrity, requiring rigorous documentation and traceability to meet audit and compliance requirements.
Global regulatory bodies like the FDA, EMA, and ICH are enforcing more rigorous standards to guarantee the quality, safety, and effectiveness of pharmaceutical products. This intensifying regulatory environment requires companies to conduct extensive analytical testing throughout all phases of drug development and production. To comply, pharmaceutical firms must implement validated testing methods, conduct comprehensive batch testing, and maintain thorough documentation to satisfy increasingly frequent audits and inspections.
Furthermore, as these companies expand globally, they must align with international regulatory frameworks, increasing the need for standardized testing practices. In response to these growing demands, both large pharma companies and smaller biotech firms are either building internal testing capabilities or turning to Contract Research Organizations (CROs) for support. Consequently, these stringent compliance requirements are playing a key role in driving continuous growth within the Pharmaceutical Analytical Testing Market.
The growing prominence of biopharmaceuticals including monoclonal antibodies, gene and cell therapies, and RNA-based drugs has added significant complexity to the drug development process. Unlike conventional small-molecule medications, these innovative therapies require highly advanced and specialized analytical testing to verify their safety, stability, and effectiveness. Analyzing biologics involves detailed structural characterization using advanced techniques like mass spectrometry and electrophoresis to examine elements such as protein structures, glycosylation, and impurities.
These therapies also require ultra-sensitive assays to measure low drug concentrations in biological fluids, rigorous stability testing due to their vulnerability to degradation, and customized testing protocols tailored to their unique biological profiles. With the continued expansion of biologic and biosimilar pipelines worldwide, the demand for sophisticated analytical services is rising particularly among Contract Research Organizations (CROs) and specialized labs equipped to handle the unique challenges of biopharmaceutical testing.
A major limitation in the pharmaceutical analytical technique market is the high expense involved in acquiring and operating sophisticated analytical instruments like HPLC, GC-MS, NMR, and LC-MS. These tools are essential for maintaining drug quality, meeting regulatory standards, and supporting drug development, but they require significant investment not only in purchasing but also in maintenance, calibration, and skilled personnel training.
This financial burden creates multiple challenges. It limits access for smaller companies and research institutions, restricting their ability to innovate and compete in the market. In low- and middle-income countries, high costs hinder broader adoption, making it difficult to align with international drug quality standards. Furthermore, the recurring expenses for consumables and servicing strain organizational resources. As a result, companies may postpone upgrading their systems or adopting newer technologies, ultimately slowing innovation and technological progress in the field.
| Report Metric | Details |
|---|---|
| Segmentations | |
| By Service Type |
Bioanalytical Testing Method Development and Validation Stability Testing Drug Substances Testing Microbial Testing Physical Characterization Others |
| By End-User |
Medical Device Companies Pharmaceutical & Biopharmaceutical Companies Others |
| Key Players |
Thermo Fisher Scientific Inc. Lonza Group LabCorp Drug Development Charles River Laboratories WuXi AppTec Eurofins Scientific Covance PRA Health Sciences SGS SA Piramal Enterprises Biocon Ltd. Medpace |
| Geographies Covered | |
| North America |
U.S. |
| Europe |
U.K. |
| Asia Pacific |
China |
| Middle East & Africa |
Saudi Arabia |
| Latin America |
Brazil |
The Pharmaceutical Analytical Testing Market is segmented by Service type and End user. Each segment plays a vital role in enhancing drug safety, ensuring regulatory compliance, and accelerating the development of high-quality pharmaceutical products.
The pharmaceutical analytical technique market is composed of various service types, each essential for ensuring the safety, effectiveness, and regulatory adherence of drug products during their development and manufacturing.
Bioanalytical testing focuses on determining drug and metabolite levels in biological samples like blood, plasma, and urine. This is vital for evaluating pharmacokinetic and pharmacodynamic profiles, as well as establishing bioequivalence in clinical and preclinical studies. With the rise in clinical trials and tightening regulatory oversight, the demand for such testing continues to grow.
Method development and validation involve creating accurate, specific, and reproducible testing methods, which are fundamental for securing regulatory approvals and maintaining consistent drug quality across production batches.
Stability testing assesses the durability of pharmaceutical products under various storage and environmental conditions, helping determine appropriate shelf life and labeling in line with international standards set by authorities like the FDA, EMA, and ICH. Drug substance testing ensures that active pharmaceutical ingredients (APIs) meet necessary quality benchmarks for identity, purity, and potency before being used in drug formulation. Microbial testing is conducted to identify contamination risks in raw materials, intermediates, and finished products, especially for sterile drugs, and is a critical component of Good Manufacturing Practice (GMP) compliance. Physical characterization examines attributes such as particle size, surface area, and polymorphism to optimize drug formulation and ensure consistent performance. The “Others” category includes niche yet crucial services such as testing for extractables and leachable, elemental impurities, and residual solvents, all of which play a role in upholding product safety and maintaining rigorous quality standards throughout the drug development lifecycle.
The pharmaceutical analytical technique market is primarily shaped by three major end-user groups. The largest segment comprises pharmaceutical and biopharmaceutical companies, driven by the need for strict quality control, regulatory compliance, and faster drug development timelines. To optimize operations and reduce expenses, these companies frequently outsource analytical testing, allowing them to concentrate on their core R&D functions.
Medical device companies are another key player, utilizing analytical testing for assessing biocompatibility, material stability, and sterility of their products. The rising complexity and regulatory requirements for combination products, such as drug-eluting stents, have increased demand in this area. The “Others” category includes contract research organizations (CROs), academic institutions, and government laboratories, all of which play a crucial role in advancing the field through method development, innovation, and collaborative research.
North America is a dominant region in the pharmaceutical analytical technique market, driven by advanced healthcare infrastructure, significant pharmaceutical and biopharmaceutical industry presence, and substantial investments in research and development. The United States, in particular, is the largest market, with an emphasis on stringent regulatory standards enforced by agencies like the FDA.
High demand for cutting-edge technologies like LC-MS, HPLC, and NMR spectroscopy for drug development and quality control further drives market growth. Additionally, the outsourcing of analytical testing services to third-party contract research organizations (CROs) is common in this region, further boosting market expansion. The rapid adoption of novel pharmaceutical products, including biologics and personalized medicine, also contributes to the growing need for advanced analytical testing services.
Europe represents another significant market for pharmaceutical analytical techniques, with countries like Germany, the UK, and France leading the way. The region is home to a strong pharmaceutical sector that benefits from robust regulatory frameworks and compliance with European Medicines Agency (EMA) standards. The demand for bioanalytical testing, stability testing, and drug substances testing is high, as companies work to meet the EU’s rigorous drug approval and quality standards. Additionally, Europe has a thriving CRO market that supports research and method development, particularly in emerging areas such as biosimilars and gene therapies. Europe’s established pharmaceutical infrastructure, coupled with the growing trend of collaboration between academic institutions and private companies, fosters continued market growth.
The Asia-Pacific region is seeing rapid growth in the pharmaceutical analytical technique market, driven by the increasing number of clinical trials, the expansion of the biopharmaceutical sector, and rising pharmaceutical production in countries like China, India, and Japan. The demand for analytical techniques in drug testing, bioanalytical testing, and microbial testing is surging, fueled by the growing healthcare needs of an expanding population. Regulatory agencies such as the National Medical Products Administration (NMPA) in China and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan are also tightening their standards, leading to increased demand for quality control and regulatory compliance testing. As the region experiences rapid industrialization and growing government investments in healthcare infrastructure, the pharmaceutical analytical market is expected to expand further, especially as India and China emerge as key manufacturing hubs for generics and biosimilars.
In Latin America, the pharmaceutical analytical technique market is experiencing steady growth, with Brazil and Mexico as the key contributors. The region is witnessing increasing demand for bioanalytical testing, stability testing, and drug substances testing, as regulatory standards continue to evolve. Latin American countries are also investing in improving their healthcare systems, which includes increasing pharmaceutical production capacity and meeting global regulatory standards. However, the market faces challenges such as economic volatility and varying regulatory frameworks across countries. Despite these challenges, the rising need for quality pharmaceuticals, particularly in the generic and over-the-counter sectors, is driving the demand for pharmaceutical analytical services in the region. The growing pharmaceutical industry, coupled with the expansion of clinical trials, is anticipated to fuel market growth in the coming years.
The pharmaceutical analytical technique market in the Middle East & Africa (MEA) is growing, albeit at a slower pace compared to other regions. In the Middle East, countries like Saudi Arabia, the UAE, and Egypt are seeing significant investments in healthcare infrastructure, which in turn is spurring demand for pharmaceutical analytical services. There is an increasing need for microbial testing, bioanalytical testing, and drug substances testing as pharmaceutical manufacturing and clinical trials ramp up in the region.
The growth of the healthcare sector in the region is also being driven by government initiatives aimed at diversifying the economy and improving healthcare access. However, the market in Africa faces challenges such as limited healthcare resources, regulatory inefficiencies, and infrastructural barriers, which can slow the adoption of advanced analytical techniques. Nonetheless, the region presents long-term growth opportunities, particularly as healthcare access improves and regulatory standards tighten.
The pharmaceutical analytical testing market was valued at USD 7.8 billion in 2024.
The pharmaceutical analytical testing market is projected to grow at a CAGR of 8.40% from 2025 to 2033.
The Bioanalytical Testing holds the largest market share.
The North America and Europe region is expected to witness the highest growth rate.
Major players include Thermo Fisher Scientific Inc., Lonza Group and LabCorp Drug Development.
1.1 Summary
1.2 Research methodology
2.1 Research Objectives
2.2 Market Definition
2.3 Limitations & Assumptions
2.4 Market Scope & Segmentation
2.5 Currency & Pricing Considered
3.1 Drivers
3.2 Geopolitical Impact
3.3 Human Factors
3.4 Technology Factors
4.1 Porters Five Forces Analysis
4.2 Value Chain Analysis
4.3 Average Pricing Analysis
4.4 M & A, Agreements & Collaboration Analysis
5.1 Pharmaceutical Analytical Testing Market, By Service Type
5.1.1 Introduction
5.1.2 Market Size & Forecast
5.2 Pharmaceutical Analytical Testing Market, By End-User
6.1 North America Pharmaceutical Analytical Testing Market, By Country
6.1.1 Pharmaceutical Analytical Testing Market, By Service Type
6.1.2 Pharmaceutical Analytical Testing Market, By End-User
6.2 U.S.
6.2.1 Pharmaceutical Analytical Testing Market, By Service Type
6.2.2 Pharmaceutical Analytical Testing Market, By End-User
6.3 Canada
7.1 U.K.
7.2 Germany
7.3 France
7.4 Spain
7.5 Italy
7.6 Russia
7.7 Nordic
7.8 Benelux
7.9 The Rest of Europe
8.1 China
8.2 South Korea
8.3 Japan
8.4 India
8.5 Australia
8.6 Taiwan
8.7 South East Asia
8.8 The Rest of Asia-Pacific
9.1 UAE
9.2 Turkey
9.3 Saudi Arabia
9.4 South Africa
9.5 Egypt
9.6 Nigeria
9.7 Rest of MEA
10.1 Brazil
10.2 Mexico
10.3 Argentina
10.4 Chile
10.5 Colombia
10.6 Rest of Latin America
11.1 Global Market Share (%) By Players
11.2 Market Ranking By Revenue for Players
11.3 Competitive Dashboard
11.4 Product Mapping